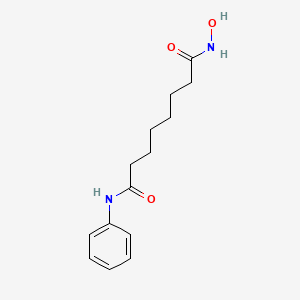
Vorinostat, 149647-78-9, SAHA, suberoylanilide hydroxamic acid, Zolinza, N-hydroxy-N'-phenyloctanediamide, N1-hydroxy-N8-phenyloctanediamide, Suberanilohydroxamic acid, MK-0683, MK0683, Vorinostat (SAHA, MK0683), Octanediamide, N-hydroxy-N'-phenyl-, OCTANEDIOIC ACID HYDROXYAMIDE PHENYLAMIDE, Vorinostat (SAHA), CCRIS 8456, N-Hyrdroxy-N'-phenyloctanediamide, NSC-701852, SHH, C14H20N2O3, CHEMBL98, MFCD00945317, NSC-748799, NSC-759852, 58IFB293JI, DTXSID6041133, CHEBI:45716, WIN64652, NSC701852, SAHA cpd, NCGC00168085-01, NCGC00168085-02, Vorinostat [USAN], Zolinza (TN) (Merck), N-hydroxy-N'-phenyl-octane-1,8-diotic acid diamide, DTXCID4021133, N'-hydroxy-N-phenyloctanediamide, Vorinostat MSD, SMR000486344, Zolinza (TN), CAS-149647-78-9, SR-05000000373, Vorinostat (JAN/USAN), Vorinostat [USAN:INN], MK 0683, vorinostatum, UNII-58IFB293JI, SKI390, HSDB 7930, 4lxz, Vorinostat(SAHA), Zolinza; SAHA, N1-hydroxy-N8-phenyl-octanediamide, SAHA, Suberoylanilide hydroxamic acid, 1zz1, VORINOSTAT [MI], SW-064652, VORINOSTAT [INN], VORINOSTAT [JAN], 8-(hydroxyamino)-8-oxo-N-phenyl-octanamide, VORINOSTAT [VANDF], cid_5311, SCHEMBL9018, VORINOSTAT [MART.], VORINOSTAT [WHO-DD], Suberoylanilidehydroxamic Acid, MLS001065855, MLS006011941, GTPL6852, VORINOSTAT [ORANGE BOOK], BDBM19149, Vorinostat (SAHA; MK0683), n-hydroxy-n'-phenyl-octanediamide, SUBERANILOHYDROXAMINIC ACID, 1t69, N-hydroxy-N''-phenyloctanediamide, BCPP000018, HMS2219L20, HMS3264D20, HMS3327C12, HMS3426G03, HMS3650D09, HMS3654G11, HMS3715E22, HMS3745M03, Pharmakon1600-01502267, BCP01858, EX-A2745, SAHA, >=98% (HPLC), Vorinostat,SAHA,Zolinza,MK-0683, Tox21_112605, Tox21_113623, Vorinostat,CAS:149647-78-9, HB1396, NSC748799, NSC759852, Octanediamide, N1-hydroxy-N8-phenyl, s1047, SK1390, AKOS015966648, Octanediamide, N1-hydroxy-N8-phenyl-, Tox21_112605_1, AC-1923, CCG-208659, CS-0589, DB02546, DG-0025, NSC 701852, NSC 748799, NSC 759852, SB17319, NCGC00168085-03, NCGC00168085-04, NCGC00168085-05, NCGC00168085-13, BP-25652, BP-30216, BV164560, HY-10221, SY009383, AM20030018, FT-0650593, H1388, NS00068618, SW199536-4, D06320, EN300-120641, AB00375377-07, AB00375377-08, AB00375377-09, AB01644613_25, A808935, Q905901, Vorinostat, SAHA, suberoylanilide hydroxamic acid, SR-05000000373-2, SR-05000000373-6, SR-05000000373-8, W-201327, BRD-K81418486-001-01-2, BRD-K81418486-001-10-3, BRD-K81418486-001-12-9, BRD-K81418486-001-13-7, BRD-K81418486-001-17-8, BRD-K81418486-001-18-6, Z1530532755, N-Hydroxy-N inverted exclamation mark -phenyloctanediamide, MK0683 , suberoylanilide hydroxamic acid , SAHA, 1227736-21-1